- Home
- News
- Lyophilized new crown nucleic acid reagent can be transported at room temperature, and can withstand 47℃ high temperature. It is no longer limited by cold chain!
Lyophilized new crown nucleic acid reagent can be transported at room temperature, and can withstand 47℃ high temperature. It is no longer limited by cold chain!
Full-component lyophilized reagents, export nucleic acid reagents to realize normal temperature transportation
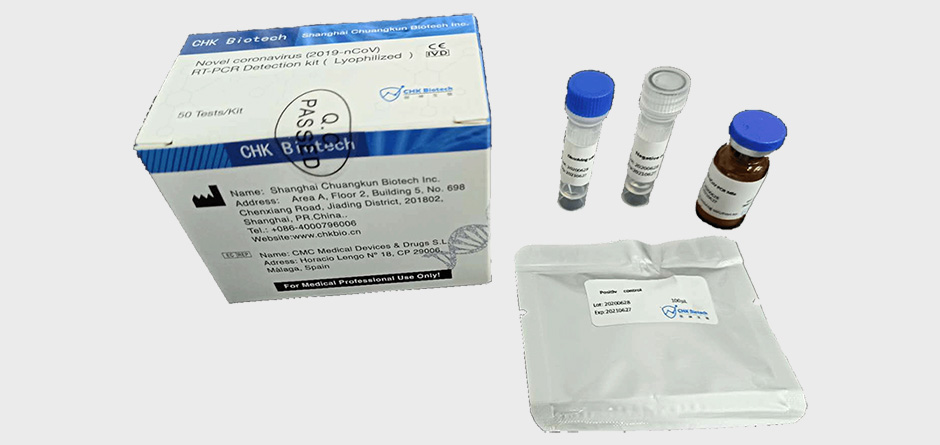
In order to break through the bottleneck that molecular diagnostic reagents need to be stored and transported at -20°C, the “Novel coronavirus 2019-nCoV RT-PCR nucleic acid detection kit (lyophilized)” developed by Shanghai Chuangkun Biotech Inc. is a full-component frozen Dry reagents have strong thermal stability to withstand high temperatures of 47°C for at least 60 days, and can be stored at room temperature and transported at room temperature. This effectively solves the pain points that the transportation of liquid COVID-19 nucleic acid reagents requires full cold chain protection in the past, and relieves the pressure on epidemic prevention and control.
Advantages of lyophilized nucleic acid reagents
Shanghai Chuangkun Biotech Inc.’s full-component lyophilized COVID-19
nucleic acid detection reagent has the following advantages in addition
to its composition and activity compared to liquid reagents:
Storage and transportation in the room temperature:it does not need to be stored at a low temperature before opening, which is convenient for medical institutions at all levels.
complete in one step:All components are lyophilized, no PCR reaction system preparation is required, and can be used after reconstitution, greatly simplifying the operation process.
Detect 3 targets at once:The target covers the Novel coronavirus ORF1a/b gene and N gene. In order to reduce false negatives, the IC test of the internal reference gene is added to the product, which can effectively monitor the entire experimental process from sampling, extraction to amplification, and avoid false negatives. Missed inspection.
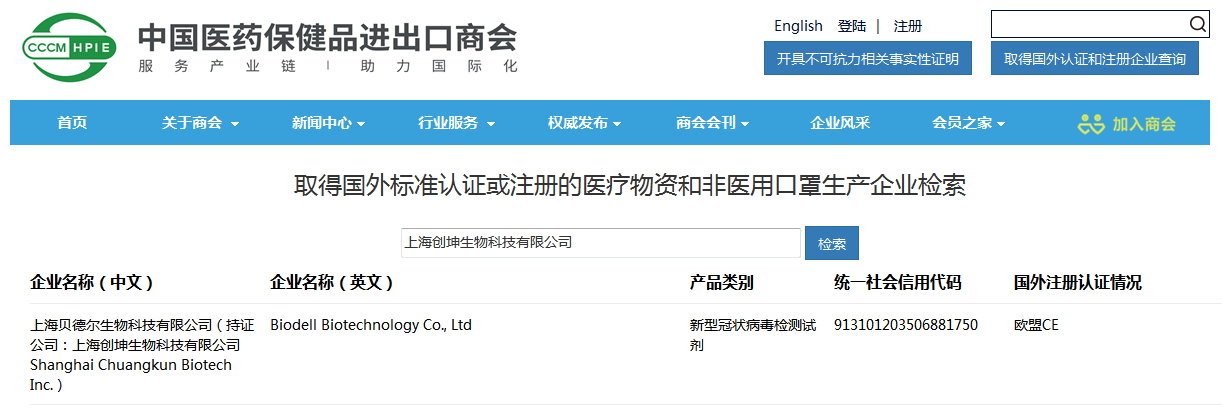
At present, the full-component lyophilized COVID-19 nucleic acid detection reagent has obtained the EU CE certification, and successfully entered the “white list” of the China Chamber of Commerce for Import and Export of Medical Insurance on September 15, 2020, and it means that the kit is officially approved to be exported for overseas sales to help the global fight the COVID-19 epidemic.

Shanghai Chuangkun Biotech Inc.has been listed in the latest “Medical Insurance Chamber of Commerce’s List of Medical Material Manufacturers Obtaining Foreign Standard Certification or Registration” issued by the China Chamber of Commerce for Import and Export of Medicines and Health Products. It has obtained export qualifications and can export to support global anti-epidemic.
The virus knows no borders, and the prevention and control of the epidemic requires the joint response of the international community. The application of the full-component freeze-drying process in the COVID-19 nucleic acid detection reagents will be beneficial to the prevention and control of the global COVID-19 epidemic. In order to support the international community to jointly respond to the global public health crisis under the COVID-19 epidemic,Shanghai Chuangkun Biotech Inc. provides high-quality “Novel coronavirus 2019-nCoV RT-PCR nucleic acid detection kit (lyophilized)” to contribute to the global fight against the epidemic Chinese power!